Innovating Non-Clinical Testing: NAMs and ESG Impact
Published Jul 21, 2025
Published 05th February 2024

Early access to medicines creates the potential for treatment using unlicenced medicines when no other treatment option is available. In the United Kingdom (UK), early access may be achieved via the Early Access to Medicines Scheme (EAMS) or ‘Specials’ as two possible options to meet patient’s unmet medical needs.
These schemes are explained below, in addition to how DLRC can help incorporate early access programs within the overall Regulatory Strategies for your product and portfolio.
There are two schemes available in the UK, Early Access to Medicines Scheme (EAMS) and ‘Specials’. The schemes vary in criteria to be met, including patient access eligibility, product licence status, regulatory process and timelines to initiate, costs, scientific assessment by the MHRA, ability to collect real world data/evidence, and involvement of other national bodies including the National Institute for Health and Care Excellence (NICE).
Patients are able to receive unlicensed medicines under the Early Access to Medicines Scheme (EAMS). This voluntary scheme aims to give patients with life threatening or seriously debilitating conditions access to medicines that do not yet have a marketing authorisation when there is a clear unmet medical need.
An application (including a fee) is made to the MHRA, which will give an opinion on the benefit/risk balance of the medicine. The process involves two steps:
First, the PIM application can be made when early clinical data indicate the medicinal product meets the designation criteria, by demonstrating significant potential benefit for patients with life-threatening or seriously debilitating conditions with high unmet need. The PIM designation will be issued after an MHRA scientific meeting.
Second, the scientific opinion describes the risks and benefits of the medicine based on submitted data which have been gathered from the patients who will benefit from the medicine. A pre-submission meeting with MHRA must be requested prior to submission of the EAMS dossier. The opinion supports the prescriber and patient to decide on whether to use the medicine before its licence is approved. The positive EAMS scientific opinion is valid for one year, with the option for renewal, and/or lapses when a marketing authorisation is granted. MHRA will publish the positive scientific opinion, a public assessment report (PAR) and the EAMS Treatment Protocol on GOV.UK website for scientific opinions.
The overall process and timeline for EAMS positive opinion can take up to 6+ months. Supply of medicines must be made available to patients free of charge. It is anticipated that real world data (RWD) / evidence (RWE) are collected during EAMS.
EAMS allows unlicensed medicines to be used in clinical practice during the later stages of the development and regulatory assessment process. If MHRA grants a positive scientific opinion (step 2 of the process), the medicine could be made available to patients 12 to 18 months before marketing authorisation (MA).
Supplementary engagement meetings can be coordinated with NICE to help ensure data collection during the EAMS period is optimised to support NICE assessment of the medicine. The EAMS Task Group will allow RWD/RWE collected during the scheme to support the NICE appraisal. If NICE recommended, EAMS products benefit from a reduced NHS England commissioning period, from 90 to 30 days following the release of NICE guidance, facilitating faster access of the product to NHS prescribers.
Medicines legislation requires that medicinal products be licensed before they are marketed in the UK. However, some patients have clinical needs that cannot be met by licensed products. The supply of unlicensed medicinal products for compassionate use is mainly provided for on the basis of the ‘named patient’ program known as the ‘Specials’ scheme. The ‘Specials’ scheme provides an exemption from needing a marketing authorisation for supply, when the product is
‘Specials’ may be requested by prescribing doctors, nurses, pharmacists, or dentists registered in the UK and may be provided free of charge or charged (generating revenue). The scheme can be used to supply products which are unlicensed in the UK and may also be unlicensed or licensed in another country. Whilst an import notification is required, MHRA scientific assessment of the product is not, hence approval for individual patient supply can be received relatively quickly (within 28 days and expedited for life-threatening circumstances). It is also possible to collect RWD / RWE during ‘Specials’ supply.
Whether patients receive unlicensed medicines via EAMS or ‘Specials,’ they are benefiting from treatment they would not otherwise receive, particularly for serious, life-threatening conditions. Prescribers play a key role in both EAMS or ‘Specials’ by identifying alternative treatments options to licensed medicines, assessing the balance of risks and potential benefits to their patients, and working with the pharmaceutical companies which supply the early access medicines. EAMS medicines are provided free of charge to the NHS (National Health Service) up to the granting of the MA. Patients receiving free of charge medicine during the EAMS period will continue to do so up to the point of a positive funding decision e.g., NICE guidance, national funding policy, local funding arrangements etc.
A key step in deciding which mechanism to use for early access in the UK depends on the medicines considered for early access. To optimise benefits, the pharmaceutical company will need to determine the value in supplying medicines prior to approval. Some key points to consider are presented below.
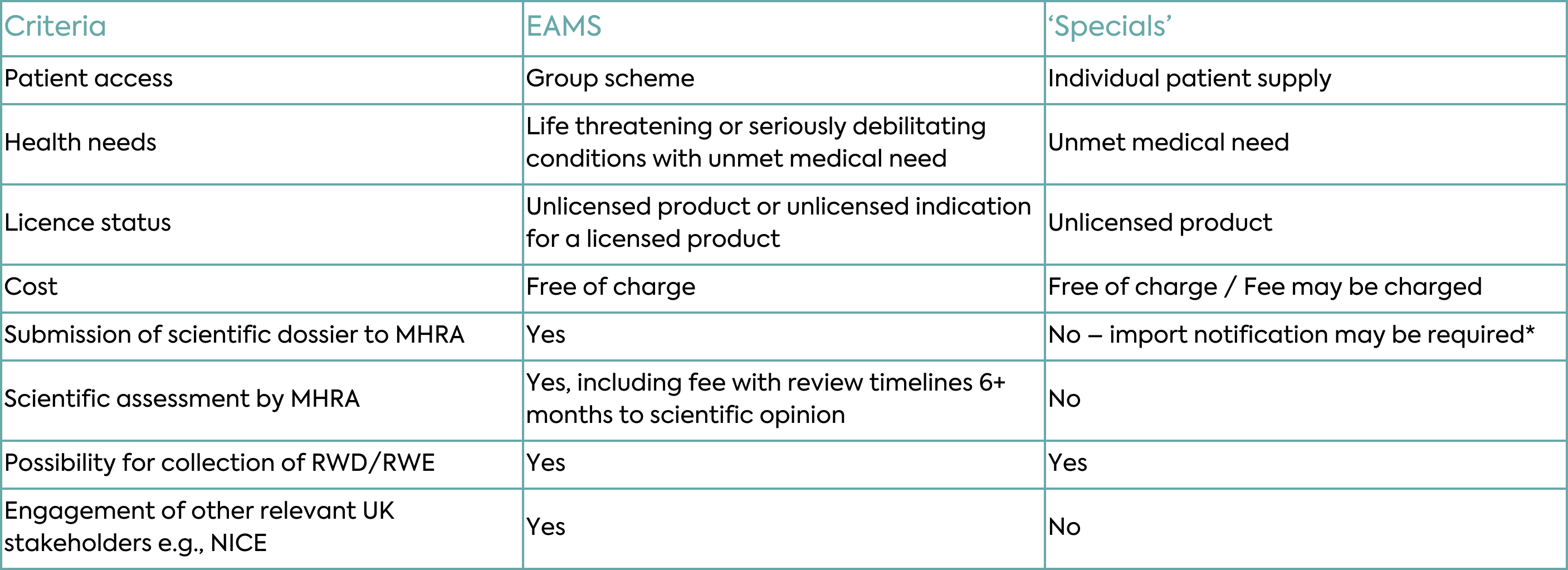 *all records in relation to specials supply should be retained by the pharmaceutical company
*all records in relation to specials supply should be retained by the pharmaceutical company
It is important that pharmaceutical companies understand it is not possible to promote unlicensed medicines in the UK. However, it is possible to provide factual responses to requests for information on a specific product (or range of products) that may be supplied.
Before early access to medicines can be initiated via EAMS or ‘Specials,’ the legal basis, eligibility criteria, and compliance requirements need to be planned and aligned with the company’s business strategy and timelines. Having a regulatory strategy aligned with the goal of supply of unlicensed medicines for the benefit of the patient, prescriber, and pharmaceutical company is essential. Understanding all the options, advantages, and potential risks, will ensure a robust product and country-specific plan from start to end of early access medicines supply. A regulatory strategy will provide a structure to ensuring compliance, adhering to regulations, and can be adapted to any changing circumstances and opportunities.
The EAMS and ‘Specials’ are two schemes which allow early access to medicines in the UK. Your company may be developing and marketing medicinal products for orphan, rare, serious, or life-threatening diseases or conditions where there are unmet medical needs. With benefits to pharmaceutical companies, the NHS, prescribers, and patients, reviewing the feasibility and creating regulatory strategy for EAMS or ‘Specials’ will help put patients’ needs first.
A forward-thinking consultancy, DLRC understands each individual company’s unique requirements for Early Access Programs and can provide the regulatory strategy to find solutions for unmet medical needs.
To discuss how early access in the UK can benefit and support your product development plans, email our regulatory experts at hello@dlrcgroup.com or via the link below.

Published Jul 21, 2025

Published Jul 11, 2025

Published Jul 07, 2025

Published May 29, 2025

Published May 29, 2025

Published May 29, 2025

Published May 29, 2025

Published May 01, 2025

Published Apr 28, 2025